
One platform, every trial. Total control.
A single, standards-based platform that transforms fragmented legacy workflows into a continuous data ecosystem, enabling real-time intelligence across the full lifecycle of RCT and RWE studies.
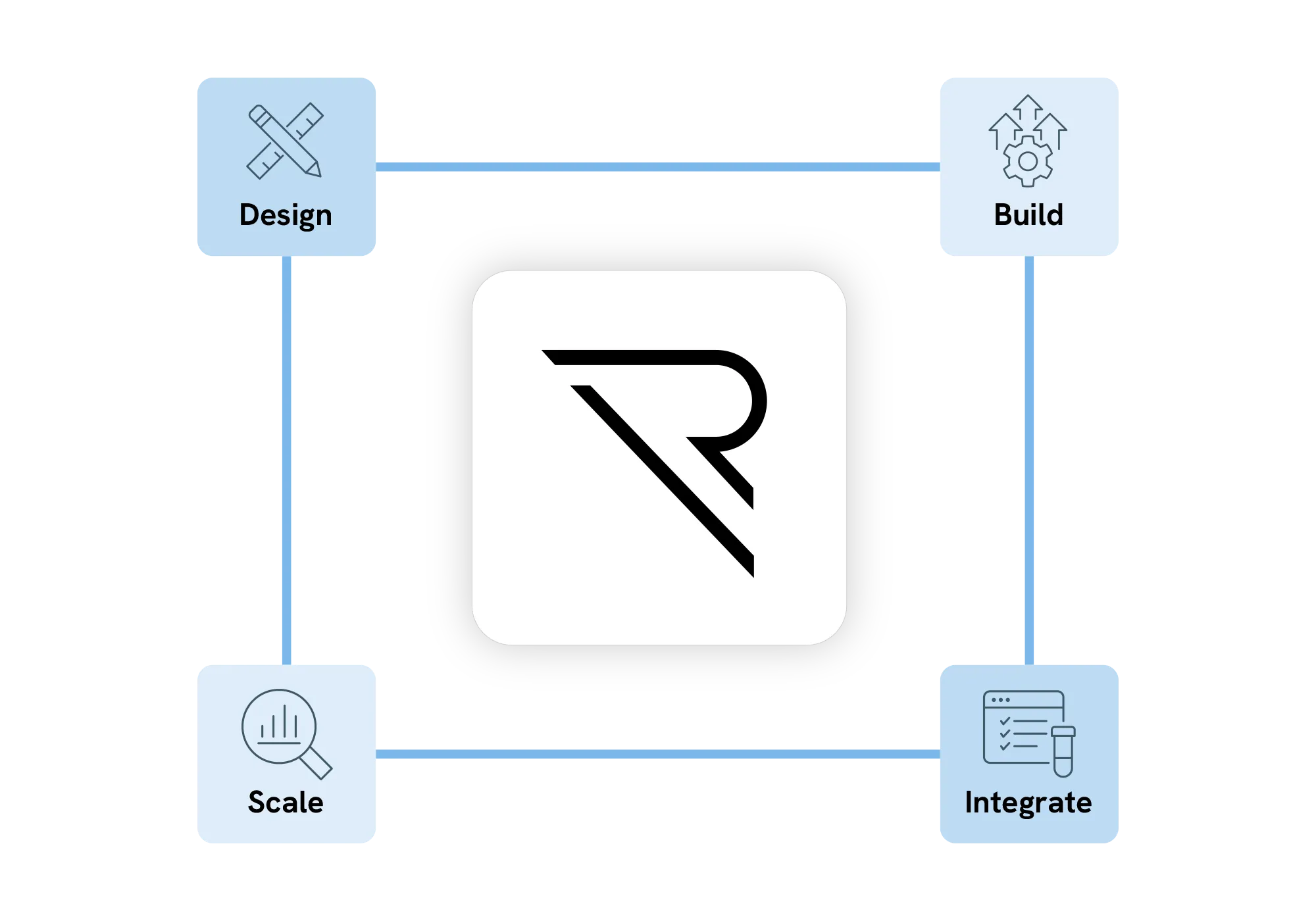
Build and design complex trials effortlessly
Design and deploy adaptive trials, registries, and everything in between. Integrate hundreds of clinical and RWD sources at a platform level while leveraging global libraries, standards, and custom functions to ensure consistency, and governance across the entire trial life-cycle.
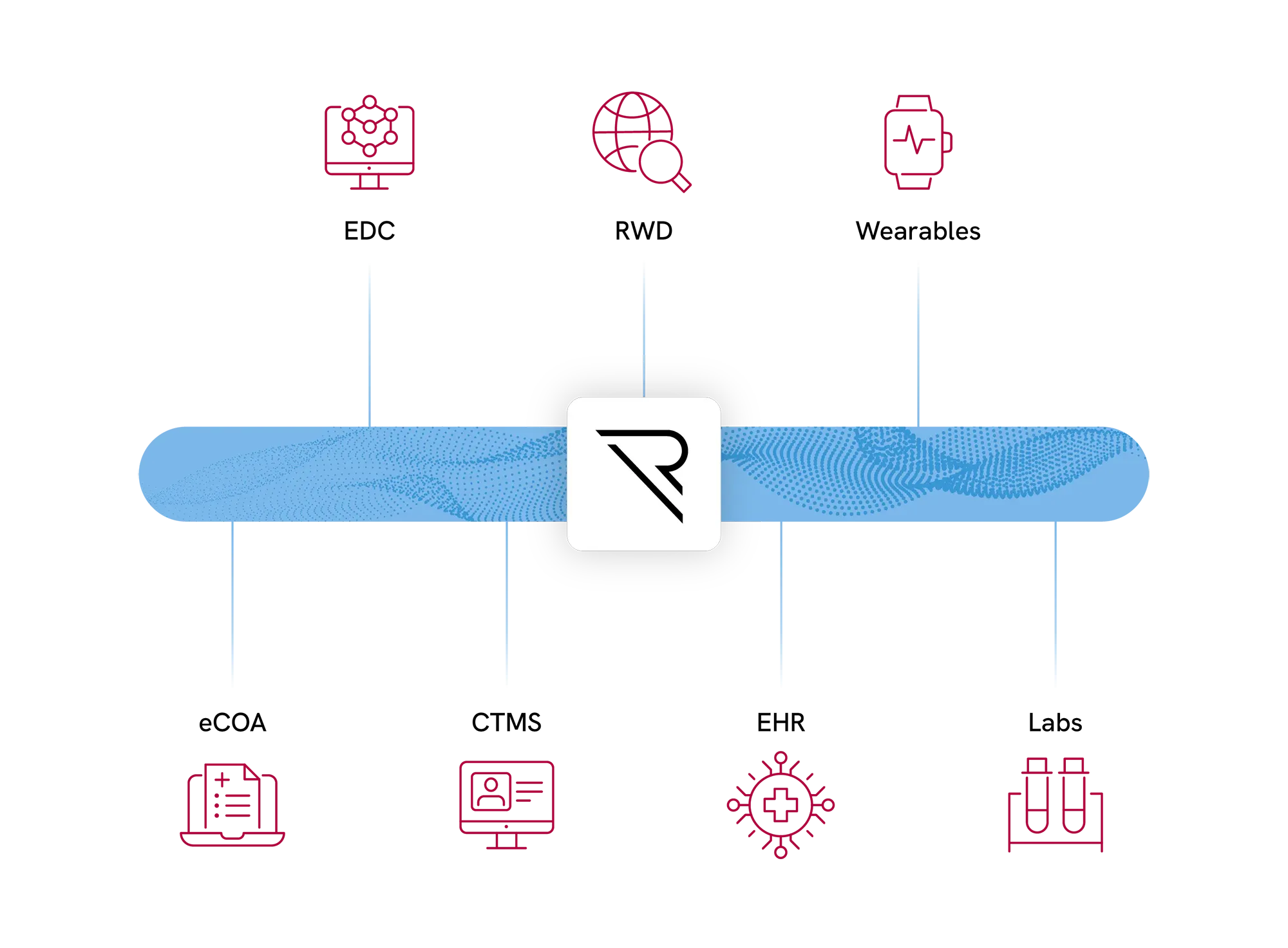
Clinical Data Hub
Capture & Manage all your Clinical Trial data. REDCap Cloud is the only platform that unifies all eCRF and non-eCRF(RWD) data at scale.
With bi-directional data flow across all sources, we help you reduce SDV costs, eliminate data silo’s, and replace the need for multiple vendors.
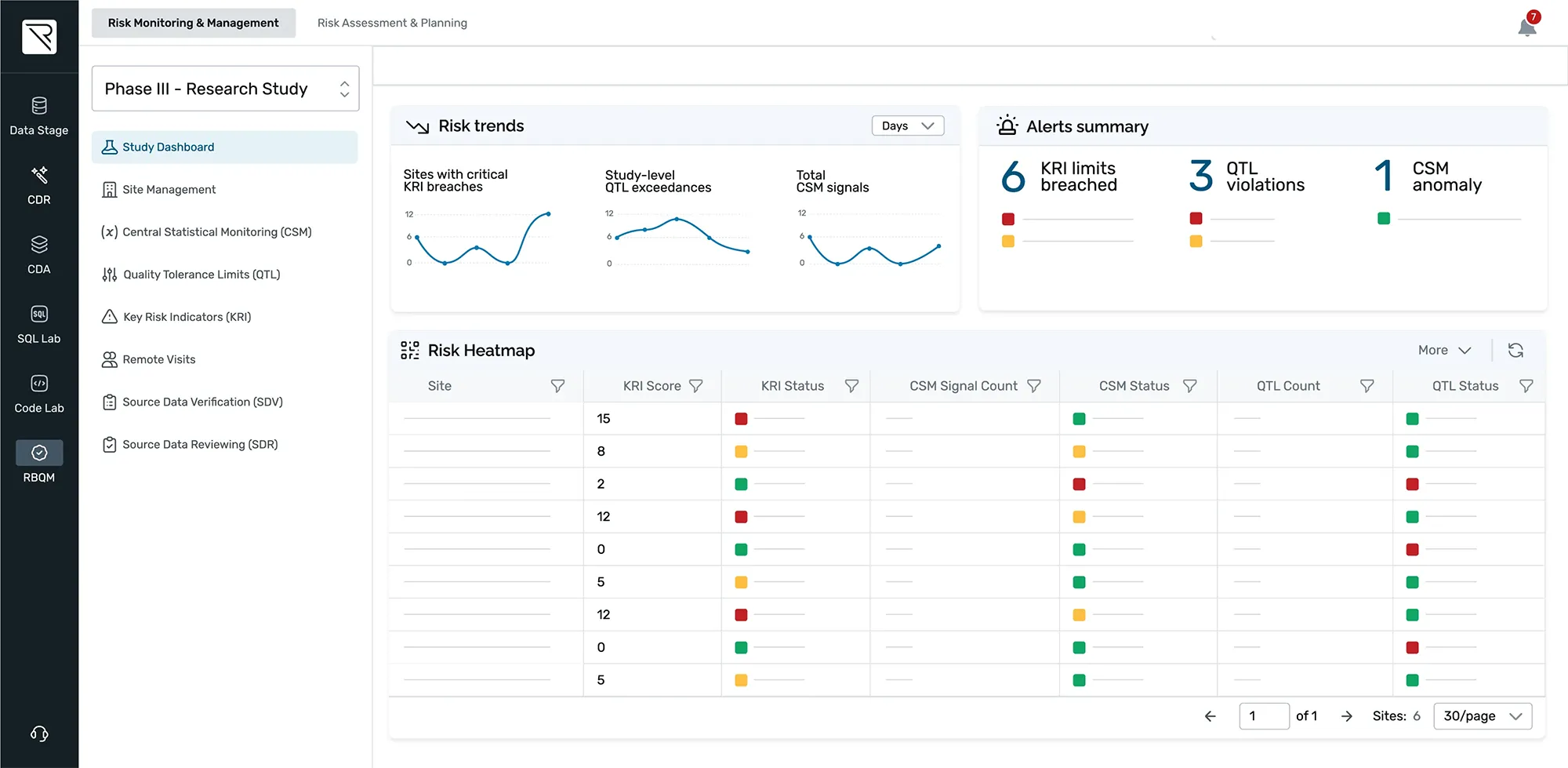
Details in the data matter
REDCap Cloud’s purpose-built analytics platform delivers real-time operational and data insights – giving you complete view of site and study performance, integrated Risk-Based Monitoring, medical monitoring, Synthetic Control Arms (Digital Twins), and on-platform AI/ML capabilities.
Focus Areas
Start simply. Scale endlessly.
One integrated clinical research platform to unify the clinical research ecosystem, delivering continuous, end-to-end data flow into real-time analytics – powering faster insights, stronger compliance and smarter decision making.
Industry
Sponsors
CROs
Medical Device, Diagnostic & DTx
Academic Research & Investigators
Health Systems
Therapeutic Areas
Oncology
Neurology & CNS
Immunology
Metabolic & GLP-1
Cardiovascular
Global security and compliance








Book a Demo →
Start and migrate your trials today – scale for tomorrows novel therapies